The eVER-HOME is an FDA-approved Type 1, HIPAA-compliant patient-level device that allows doctors to monitor their patients remotely. Patients can regularly record vital test data using bluetooth-enabled devices, and the data is transmitted directly to their clinician. It includes patient notifications, compliance alerts, and virtual exam capability.
Research
The development of the patient-level device came from the clinical-device, the IDM-100. Like that one, a requirement for this device was to re-use as many elements as possible. All the interface elements, icons and test device images, and UX were adapted from the IDM100 development.
One major difference was the screen-density differences between the tablets — the IDM100 used MDPI on a 10" tablet, one of the new tablets used HDPI on a 10" display, and the other used HDPI on an 8" display. I worked out the differences for different screen densities to present to the development team.
Layouts for different device resolution densities.
Planning
Again, I produced a sitemap and wireframes for myself. The management team was not interested in reviewing these items.
Page Map
Home page wireframe
patient info wireframe
Visual Design
The different screen sizes and densities meant that screen elements had to scale differently, shown here in Display Units (DP).
1280x800DP @ MDPI scale
853x533dp @ HDPI scale
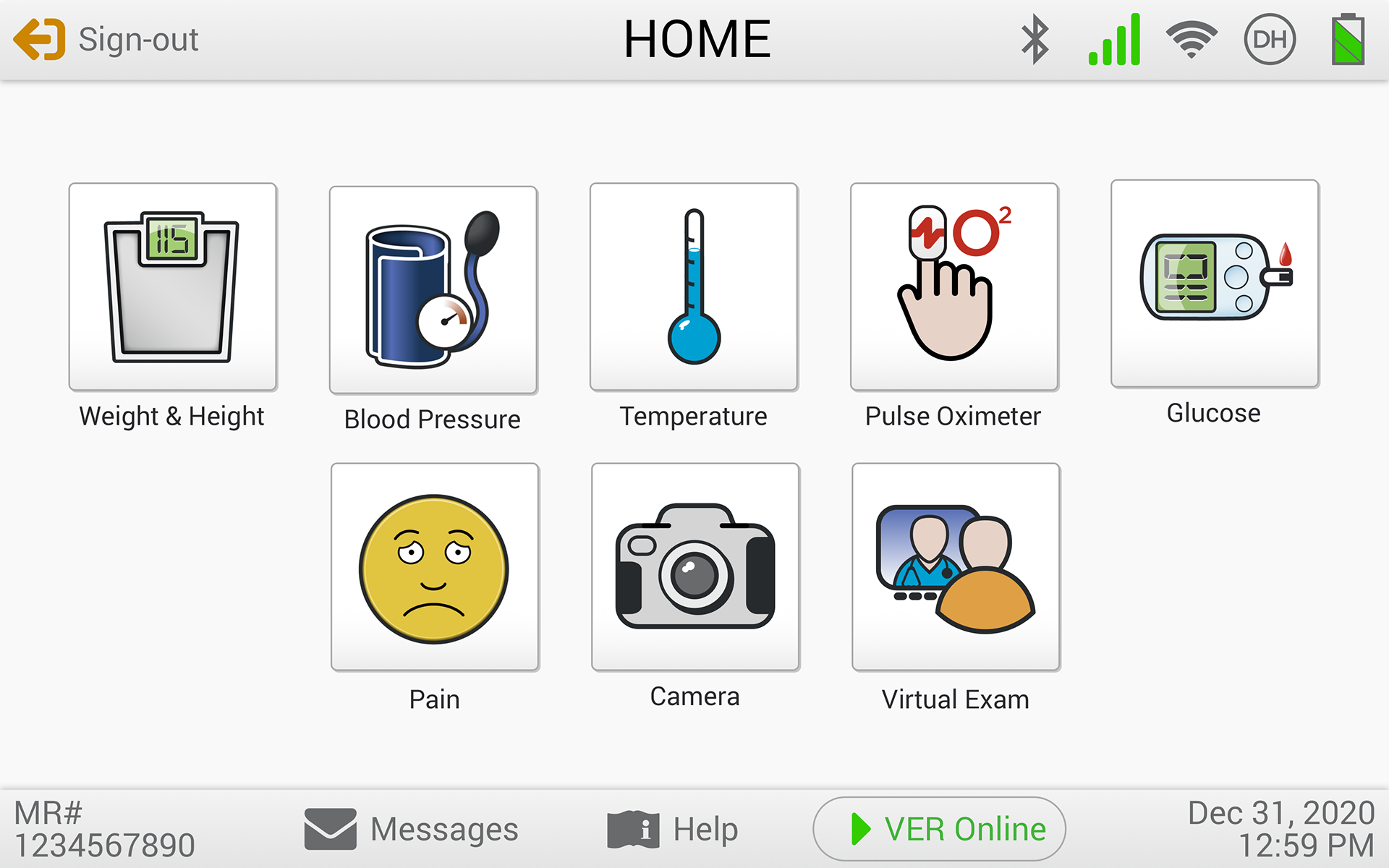
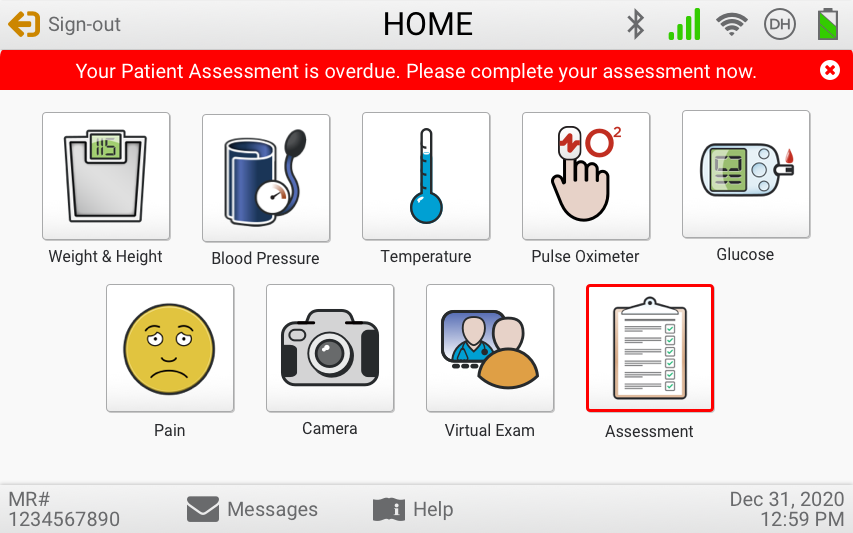
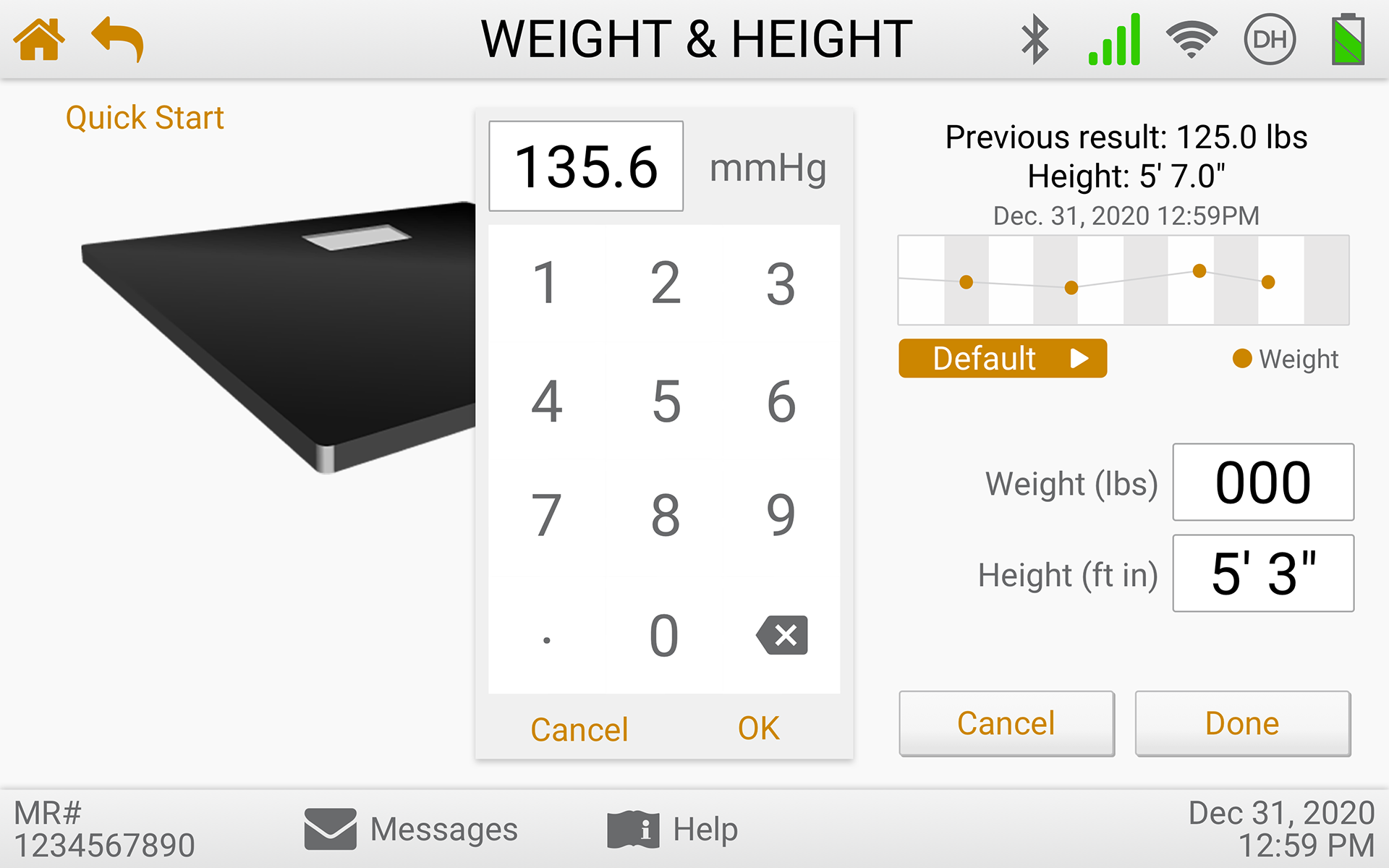
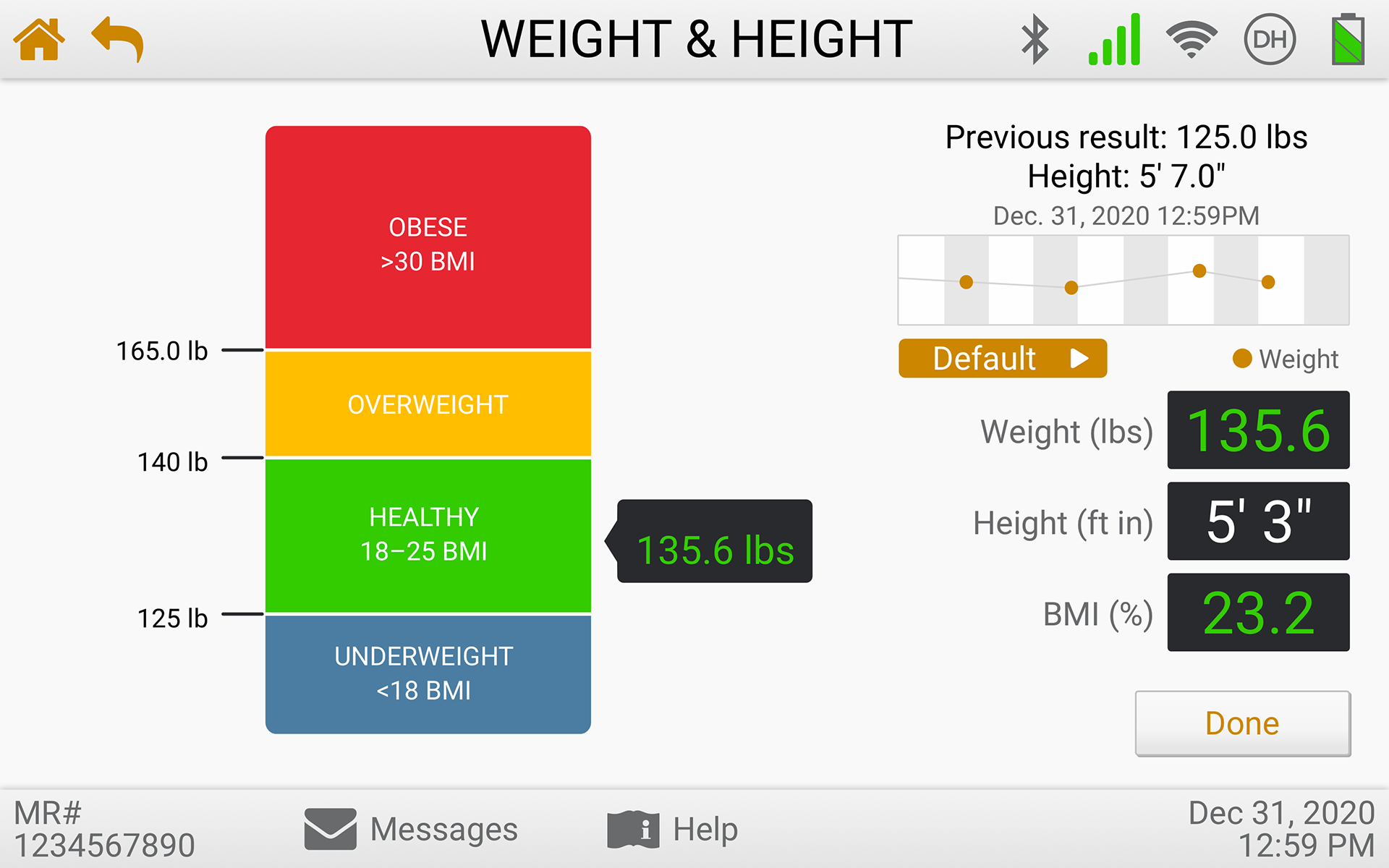
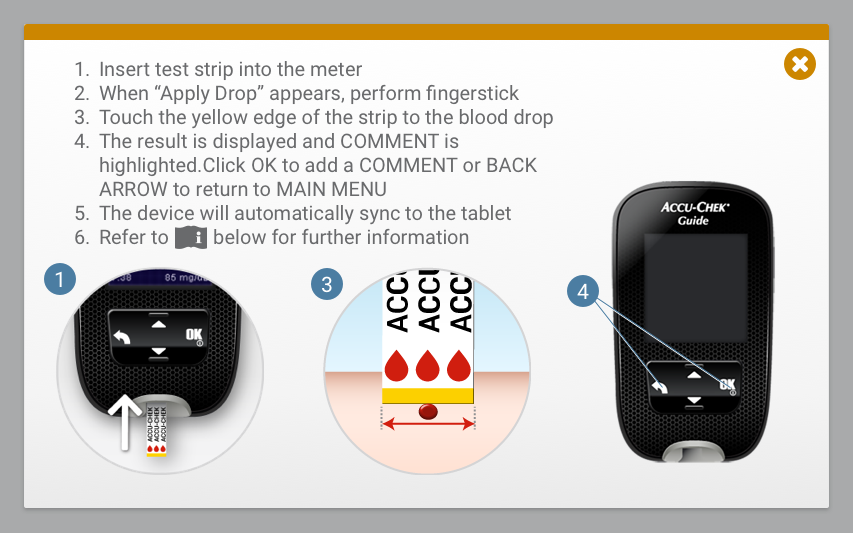
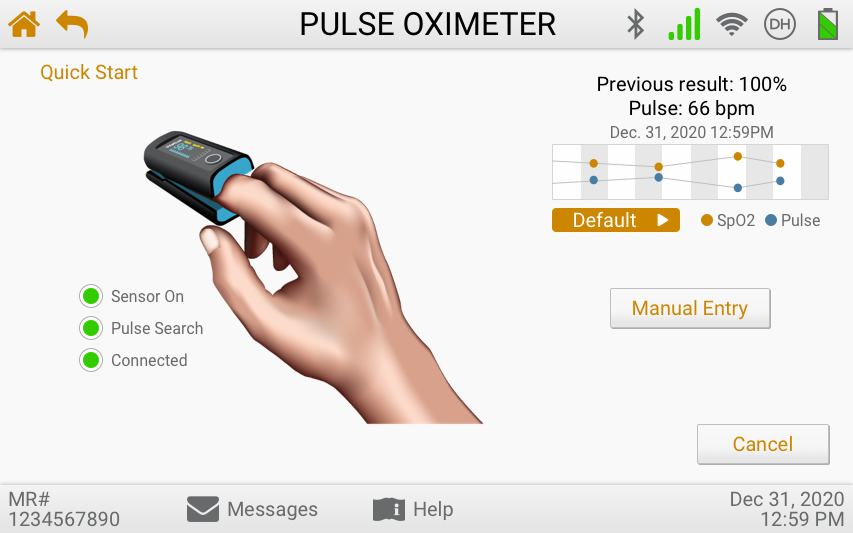


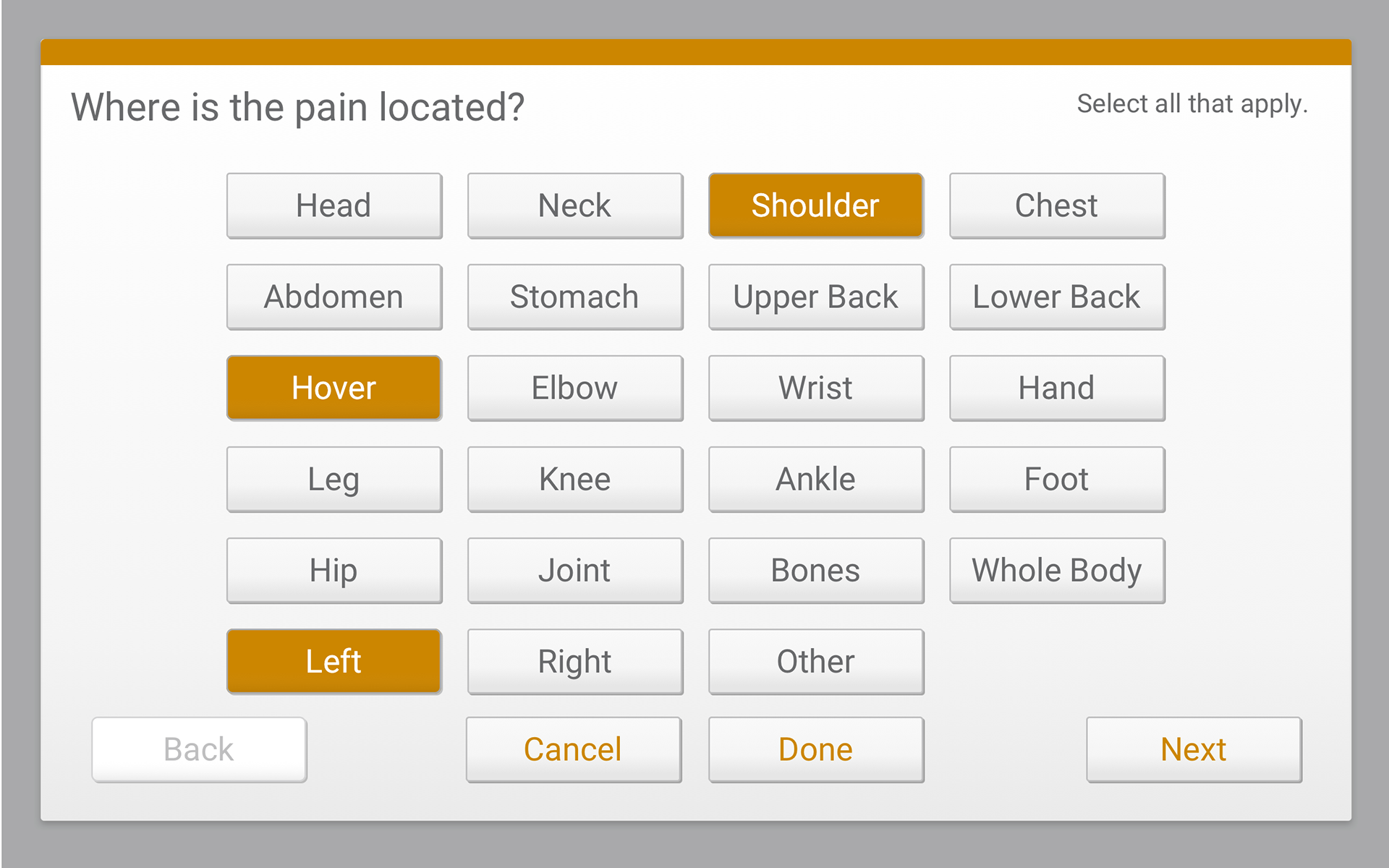
Usability Testing
A single usability survey was conducted to satisfy regulatory requirements. Using process and elements from Usability.gov, A group of 5 friends and family were asked to perform vitals tests using the eVER-HOME and provide feedback on their experience.
As expected, the majority of them didn't find any issue. There was one good observation about not being able to find Help when they needed it, but the feedback was never acted on.